Pneumococcal Infections Clinical Trial Analysis: Key Insights into Rich Pipeline Featuring 15+ Companies and 20+ Therapies | DelveInsight
The pneumococcal infection market is poised for steady growth, driven by a combination of high global disease burden, rising antimicrobial resistance, and expanded immunization initiatives. Analyst sentiment remains positive as widespread public health programs and government-backed vaccination campaigns, particularly in developing regions, continue to boost vaccine uptake. The emergence of next-generation pneumococcal vaccines with broader serotype coverage is expected to enhance market competitiveness and address unmet needs. Additionally, the growing elderly population, especially in high-income countries, further underscores long-term demand, positioning the market for continued expansion amid evolving healthcare priorities.
New York, USA, Aug. 21, 2025 (GLOBE NEWSWIRE) -- Pneumococcal Infections Clinical Trial Analysis: Key Insights into Rich Pipeline Featuring 15+ Companies and 20+ Therapies | DelveInsight
The pneumococcal infection market is poised for steady growth, driven by a combination of high global disease burden, rising antimicrobial resistance, and expanded immunization initiatives. Analyst sentiment remains positive as widespread public health programs and government-backed vaccination campaigns, particularly in developing regions, continue to boost vaccine uptake. The emergence of next-generation pneumococcal vaccines with broader serotype coverage is expected to enhance market competitiveness and address unmet needs. Additionally, the growing elderly population, especially in high-income countries, further underscores long-term demand, positioning the market for continued expansion amid evolving healthcare priorities.
DelveInsight’s 'Pneumococcal Infections Pipeline Insight 2025' report provides comprehensive global coverage of pipeline pneumococcal infections therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the pneumococcal infections pipeline domain.
Key Takeaways from the Pneumococcal Infections Pipeline Report
- DelveInsight’s pneumococcal infections pipeline report depicts a robust space with 15+ active players working to develop 20+ pipeline pneumococcal infections drugs.
- Key pneumococcal infections companies such as Gangagen, CanSino Biologics Inc., GlaxoSmithKline, Panacea Biotech, Vaxcyte, Sanofi, BioVersys AG, Inventprise Inc., Beijing Zhifei Lvzhu Biopharmaceutical, GPN Vaccines, Virometix AG, Abera Bioscience AB, and others are evaluating new pneumococcal infections drugs to improve the treatment landscape.
- Promising pipeline pneumococcal infections therapies, such as klebicins, PBPV, GSK5101955, NuVac-23, VAX 24, GBP410, BV100, IVT PCV-25, 26-valent pneumococcal conjugate vaccine, Gamma-PN3, V-212, Ab-01.12, and others, are in different phases of pneumococcal infections clinical trials.
- In July 2025, SK bioscience announced that the company and its co-development partner, Sanofi, had received regulatory approval to initiate clinical trials in a pediatric population in China of its 21-valent pneumococcal conjugate vaccine (PCV) candidate, GBP410. This approval marks a key step toward entering one of the world’s most strategically important vaccine markets.
- In February 2025, Vaxcyte, Inc. announced that the first study participants have been dosed in the second and final stage of the ongoing Phase II study of VAX-31 in healthy infants. Advancement to Stage II follows a blinded assessment of the Stage 1 safety and tolerability data per the study protocol. This study is evaluating the safety, tolerability and immunogenicity of VAX-31, a 31-valent pneumococcal conjugate vaccine (PCV) candidate designed to prevent invasive pneumococcal disease (IPD), in healthy infants.
- In December 2024, Sanofi and SK bioscience entered into a new chapter of their collaboration in pneumococcal vaccines with an expanded agreement to develop, license and commercialize next-generation PCVs for both pediatric and adult populations, reaffirming their commitment to fighting pneumococcal disease. Under the terms of the expanded agreement, both companies will co-fund research and development costs.
- In November 2024, Vaxcyte, Inc. announced positive regulatory updates, including the United States Food and Drug Administration (FDA) clearance of the VAX-31 infant Investigational New Drug (IND) application and the FDA granting Breakthrough Therapy designation (BTD) for VAX-31 for the prevention of invasive pneumococcal disease (IPD) in adults.
- In October 2024, BioVersys AG, announced the completion of the last patient's last visit in the Phase II clinical trial with its lead asset BV100, the company's novel formulation of rifabutin suitable for intravenous administration. The Phase II multicenter, open label, randomized, active controlled study evaluated the pharmacokinetics, efficacy and safety of BV100 in adult patients with ventilator associated bacterial pneumonia (VABP) suspected or confirmed to be due to Carbapenem Resistant Acinetobacter baumannii (CRAB).
- In September 2024, Vaxcyte, Inc. announced positive topline results from its Phase I/II study of VAX-31, a 31-valent pneumococcal conjugate vaccine (PCV) candidate. The study, which involved 1,015 adults aged 50 and older, found that VAX-31 was well tolerated and elicited strong opsonophagocytic activity (OPA) immune responses for all 31 serotypes evaluated.
- In January 2024, Inventprise Inc. announced the completion of vaccination in their Phase II dose ranging study of its 25-valent pneumococcal conjugate vaccine (IVT PCV-25) in young adults. The study is being conducted in four sites in Canada. This vaccine is designed to help prevent pneumococcal disease caused by serotypes not covered in the current PCVs and to help provide protection to people globally, including in low- and middle-income regions where the pneumococcal disease burden is greatest.
Request a sample and discover the recent advances in pneumococcal infections drugs @ Pneumococcal Infections Pipeline Report
The pneumococcal infections pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage pneumococcal infections drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the pneumococcal infections clinical trial landscape.
Pneumococcal Infections Overview
Pneumococcal infections are caused by the bacterium Streptococcus pneumoniae, which can lead to a range of illnesses, from mild respiratory infections to severe invasive diseases. The bacteria are commonly found in the upper respiratory tract and may not always cause illness; however, they can become pathogenic under certain conditions. Pneumococcal infections spread through respiratory droplets when an infected person coughs or sneezes, and they are more common in young children, older adults, and individuals with weakened immune systems.
The symptoms of pneumococcal infections vary depending on the affected organ system. In cases of pneumonia, patients may experience cough, chest pain, difficulty breathing, fever, and chills. Meningitis caused by S. pneumoniae can result in headache, stiff neck, confusion, and sensitivity to light. Otitis media, another common manifestation, presents with ear pain, fever, and irritability in children. Sinusitis and bloodstream infections are other possible clinical presentations.
Diagnosis of pneumococcal infections typically involves clinical evaluation supported by laboratory tests. Common diagnostic tools include chest X-rays for pneumonia, blood cultures, cerebrospinal fluid analysis for meningitis, and sputum cultures to identify the bacteria. Rapid antigen detection tests and polymerase chain reaction (PCR) assays are also used to confirm the presence of S. pneumoniae, especially in severe or invasive cases.
Treatment usually involves antibiotic therapy, with penicillin or amoxicillin being first-line treatments for susceptible strains. However, due to emerging antibiotic resistance, broader-spectrum antibiotics like ceftriaxone or vancomycin may be required in some cases. Supportive care, such as hydration, oxygen therapy, and fever management, is also important. Vaccination with pneumococcal conjugate vaccines (PCV13, PCV15, PCV20) and pneumococcal polysaccharide vaccine (PPSV23) plays a crucial role in preventing pneumococcal disease, especially in high-risk populations.
Find out more about pneumococcal infections drugs @ Pneumococcal Infections Treatment
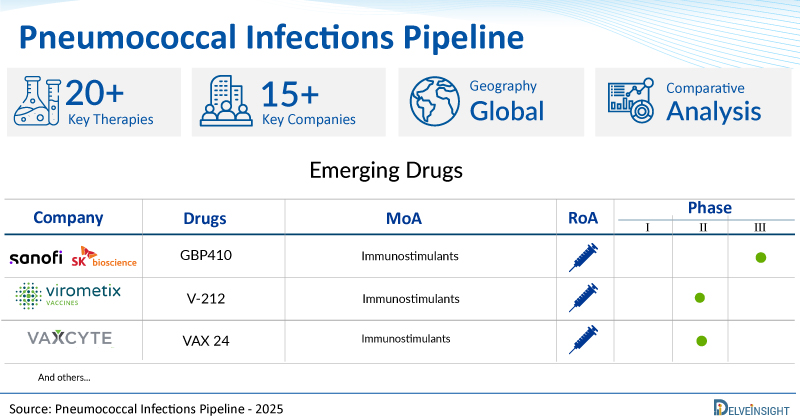
A snapshot of the Pipeline Pneumococcal Infections Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| GBP410 | Sanofi/ SK Bioscience | III | Immunostimulants | Intramuscular |
| V-212 | Virometix | II | Immunostimulants | Intramuscular |
| VAX 24 | Vaxcyte | II | Immunostimulants | Intramuscular |
Learn more about the emerging pneumococcal infections therapies @ Pneumococcal Infections Clinical Trials
Pneumococcal Infections Therapeutics Assessment
The pneumococcal infections pipeline report proffers an integral view of the emerging pneumococcal infections therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Pneumococcal Infections Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intranasal, Intrathecal, Intravenous, Oral, Parenteral, Subcutaneous, Intramuscular, Transdermal
- Therapeutics Assessment By Molecule Type: Antisense oligonucleotide, Gene therapy, Hormones, Neuropeptides, Oligonucleotides, Small molecule, Triglyceride
- Therapeutics Assessment By Mechanism of Action: Immunostimulants
- Key Pneumococcal Infections Companies: Gangagen, CanSino Biologics Inc., GlaxoSmithKline, Panacea Biotech, Vaxcyte, Sanofi, BioVersys AG, Inventprise Inc., Beijing Zhifei Lvzhu Biopharmaceutical, GPN Vaccines, Virometix AG, Abera Bioscience AB, and others.
- Key Pneumococcal Infections Pipeline Therapies: klebicins, PBPV, GSK5101955, NuVac-23, VAX 24, GBP410, BV100, IVT PCV-25, 26-valent pneumococcal conjugate vaccine, Gamma-PN3, V-212, Ab-01.12 and others.
Dive deep into rich insights for new pneumococcal infections treatments, visit @ Pneumococcal Infections Drugs
Table of Contents
| 1. | Pneumococcal Infections Pipeline Report Introduction |
| 2. | Pneumococcal Infections Pipeline Report Executive Summary |
| 3. | Pneumococcal Infections Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Pneumococcal Infections Clinical Trial Therapeutics |
| 6. | Pneumococcal Infections Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Pneumococcal Infections Pipeline: Late-Stage Products (Phase III) |
| 8. | Pneumococcal Infections Pipeline: Mid-Stage Products (Phase II) |
| 9. | Pneumococcal Infections Pipeline: Early-Stage Products (Phase I) |
| 10. | Pneumococcal Infections Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Pneumococcal Infections Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Pneumococcal Infections Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the pneumococcal infections pipeline therapeutics, reach out @ Pneumococcal Infections Therapeutics
Related Reports
Pneumonia, Pneumococcal Pipeline
Pneumonia, Pneumococcal Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key pneumonia, pneumococcal companies, including Biotest AG, Vaxcyte, Inc., Eubiologics Ltd., Eagle Pharmaceuticals, GlaxoSmithKline, among others.
Bacteremia Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key bacteremia companies, including Merck & Co., Inc, Ronak Daru Co., Bayer AG, Pfizer Inc., Baxter, ANI Pharmaceuticals, Inc., Theravance Biopharma, Novartis AG, Fresenius SE & Co. KGaA, Mylan N.V., Sun Pharmaceutical Industries Ltd., among others.
Bacteremia Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key bacteremia companies, including XBiotech, LegoChem Biosciences, Basilea Pharmaceutica, ContraFect, Merck & Co., Cumberland Pharmaceuticals, Theravance Biopharma, Entasis Therapeutics, Melinta Therapeutics, GlaxoSmithKline, among others.
Klebsiella Pneumoniae Infections Pipeline
Klebsiella Pneumoniae Infections Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Klebsiella pneumoniae infections companies, including Locus Biosciences, Inc., Inventprise, Limma Tech Biologics, among others.
Staphylococcus aureus Bacteremia Pipeline
Staphylococcus aureus Bacteremia Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Staphylococcus aureus bacteremia companies, including ContraFect, Armata Pharmaceuticals, iNtRON Biotechnology, Durata Therapeutics, Cipher Pharmaceuticals, XBiotech, Basilea Pharmaceutica, Genentech, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
